Our approach
We employ well-defined model systems that allow fundamental insights, as well as increasingly complex materials under realistic reaction conditions that provide the transfer to the application. While vacuum-based deposition and surface preparation techniques yield highest definition surfaces, wet-chemical preparation approaches enable nano-structuring.
These materials are investigated by surface sensitive (ex situ, in situ, and operando) spectroscopies and structural methods providing insights into the interphase composition, chemical interactions and electronic structure, specifically at these interfaces. Complementary electrochemical techniques allow for activity and stability assessment, as well as insight into reaction mechanisms.

Recent Highlights
-
![]()
![]()
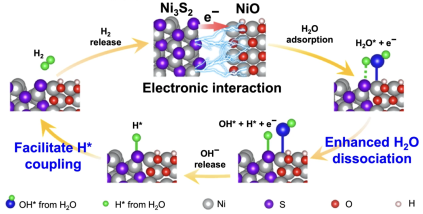
Study on Operando Transformation of NiS Electrocatalysts in the HER published
2024/06/26
What causes the increased reactivity of transition metal chalcogenides? Using operando spectroscopy, the restructuring of NiS electrocatalysts into a Ni3S2|NiO system could be demonstrated. This interface can also be held responsible for increased reactivity on a mechanistic level. Find out more here.
-
![]()
![]()
Review on Multijunction Absorbers for Artificial Leaf Photoelectrodes Published
2024/04/26
Learn in this comprehensive overview how multilayer absorbers for artificial leaves enable a competitive and sustainable photocatalyst for water splitting, how to design these structures and what urgent research questions remain to be answered.
-
![]()
![]()
Publication on the stability of the OER-active phase of NiOx-based electrocatalysts now online
2024/01/30
Can the NiOOH active phase of NiOx-based electrocatalysts be studied by ex situ techniques? To adress this question, the time-stability of the phase was studied by combining operando Raman spectroscopy with ex situ x-ray photoelectron spectroscopy.
Current Projects
-
![]()
![]()
Priority Program SPP2080 DynaKat – Project DaCapo
2023/01/01
Dynamic operation of Rutile-based oxygen evolving electrocatalysts
In the DaCapo project, the dynamic operation of electrocatalysts for the water splitting reaction, specifically the oxygen evolution reaction, is investigated as a means of improving the hydrogen production efficiency. As model system, Iridium oxide, ruthenium oxide, and mixtures thereof are chosen. In acidic electrolytes they show a high efficiency and sufficient stability to be employed at the industrial scale.
-
![]()
![]()
CRC 1487 Iron, upgraded – Project C08
2022/03/01
Electronic Structure and Redox Properties of Iron in Different Environments Studied by Valence and Core Level Spectroscopies
The electronic properties of iron such as the band structure, work function, ionization potential and electron affinity, which determine its redox properties in solid state and liquid phase reactions, sensitively depend on the chemical, coordinating environment of the Fe species in question.
-
![]()
![]()
BMBF H2Giga PrometH2eus
2021/01/01
Characterization and design of inner and outer interfaces in O2-evolving electrodes for alkaline water splitting
The BMBF is currently funding the flagship project H2Giga, which aims at bringing innovative and more efficient electrolysers into series production. Within that project, the PrometH2eus consortium combines fundamental and applied science to synthesize, evaluate and optimize new materials for the oxygen evolution reaction in alkaline electrolysis.