Cation reordering instead of phase transitions: Origins and implications of contrasting lithiation mechanisms in 1D ζ- and 2D α-V2O5
New Publication in “Proceedings of the National Academy of Sciences”
2022/02/01
Authors: Yuting Luo, Shahed Rezaei, David A. Santos, Yuwei Zhang, Joseph V. Handy, Luis Carrillo, Brian J. Schultz, Leonardo Gobbato, Max Pupucevski, Kamila Wiaderek, Harry Charalambous, Andrey Yakovenko, Matt Pharr, Bai-Xiang Xu, and Sarbajit Banerjee
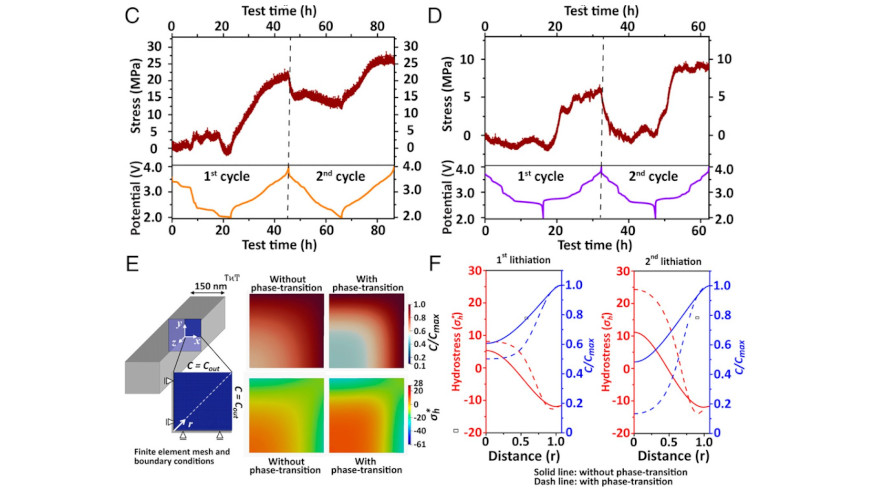
Substantial improvements in cycle life, rate performance, accessible voltage, and reversible capacity are required to realize the promise of Li-ion batteries in full measure. Here, we have examined insertion electrodes of the same composition (V2O5) prepared according to the same electrode specifications and comprising particles with similar dimensions and geometries that differ only in terms of their atomic connectivity and crystal structure, specifically two-dimensional (2D) layered α-V2O5 that crystallizes in an orthorhombic space group and one-dimensional (1D) tunnel-structured ζ-V2O5 crystallized in a monoclinic space group. By using particles of similar dimensions, we have disentangled the role of specific structural motifs and atomistic diffusion pathways in affecting electrochemical performance by mapping the dynamical evolution of lithiation-induced structural modifications using ex situ scanning transmission X-ray microscopy, operando synchrotron X-ray diffraction measurements, and phase-field modeling. We find the operation of sharply divergent mechanisms to accommodate increasing concentrations of Li-ions: a series of distortive phase transformations that result in puckering and expansion of interlayer spacing in layered α-V2O5, as compared with cation reordering along interstitial sites in tunnel-structured ζ-V2O5. By alleviating distortive phase transformations, the ζ-V2O5 cathode shows reduced voltage hysteresis, increased Li-ion diffusivity, alleviation of stress gradients, and improved capacity retention. The findings demonstrate that alternative lithiation mechanisms can be accessed in metastable compounds by dint of their reconfigured atomic connectivity and can unlock substantially improved electrochemical performance not accessible in the thermodynamically stable phase.



