Photoelectrochemical water splitting / Solar fuels
Energy storage is one of the most important topics for a successful use of renewable energy from wind and from the sun in the future. An ideal system would mimic the natural photosynthesis cycle, but with a much higher efficiency. Hydrogen is one of the most promising candidates in this respect, since it could be produced from water by electrolysis, stored, and then combusted again to water and energy without the production of any waste and any carbon dioxide.
The direct production of hydrogen by water splitting using solar light has theoretical efficiencies of up to 20% using single bandgap semiconductors and more than 30% can be reached using advanced photovoltaic converters, e.g. a combination of two semiconductor materials. In the laboratory efficiencies of more than 12% and 18% have been proven for single or tandem semiconductor devices, respectively. Still the used materials are too expensive or do not possess a long-time stability.
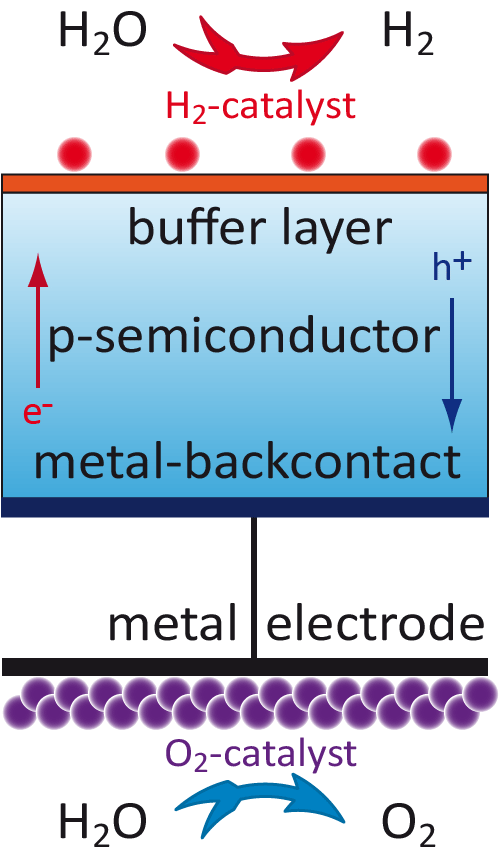

To overcome these problems we investigate thin film semiconductor devices produced from cheap and abundant materials to achieve an efficient light absorption and charge separation. For an effective electronic coupling between the solid absorber and the liquid electrolyte the interface layer and for the charge transport between the absorber and the reacting redox species in the solution metal nanoparticles as highly efficient catalysts have to be optimized separately.






