Large-scale hydrogen production
Teams at TU Darmstadt are carrying out research as part of the lead project H2Giga
2021/09/07 by Silke Paradowski
Green hydrogen produced using energy from renewable sources is one of the most important energy carriers for the energy transition in Germany. In order to support the hydrogen economy, the German Federal Ministry for Education and Research (BMBF) has initiated several lead projects. Four teams at TU Darmstadt are participating in the lead project H2Giga and adding their expertise to the development of the technology that is required for the large-scale production of hydrogen.
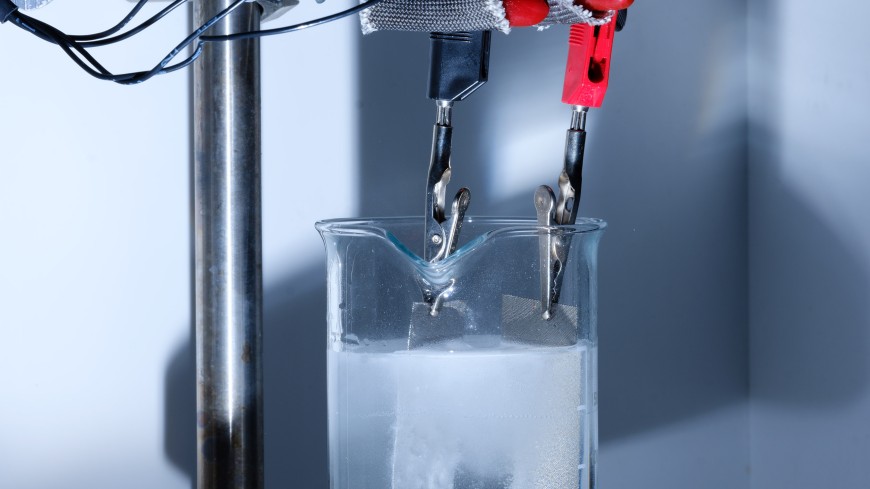
Researchers in four research groups at TU Darmstadt are participating in the H2Giga project PrometH2eus and have received total funding of around 2.9 million euros. Within the framework of the overarching lead project, they are helping to make novel and efficient electrolysers for the production of hydrogen from renewable energies ready for series production.
These devices for splitting water into its elementary components of hydrogen and oxygen must be robust and scalable, modular so that they can be adapted to their respective installation location and above all capable of producing hydrogen on an industrial scale. This is because the aim of the National Hydrogen Strategy is to develop five gigawatts of electrolysis capacity by 2030. The H2Giga project PrometH2eus will play an important role in achieving this goal. The main task is the application-oriented development of catalysts and electrodes for alkaline water electrolysis. Next generation catalyst materials have usually only been produced up to now on a laboratory scale and tested under conditions that prohibits a direct upscale for industrial application.
PrometH2eus combines basic research with application research. In the synthesis, evaluation and optimisation of new materials, the researchers not only plan to gain important basic knowledge but also to directly focus on the technical feasibility of the solutions.
“Splitting water into hydrogen and oxygen is a really important step for a modern energy cycle. In these four research projects, TU Darmstadt is demonstrating that it has the expertise and willingness required to deliver solutions for a reliable and sustainable energy supply in the near future”, explains Professor Barbara Albert, Vice President for Research and Early Careers at TU Darmstadt.
In these four research projects, TU Darmstadt is demonstrating that it has the expertise and willingness required to deliver solutions for a reliable and sustainable energy supply in the near future.
Research at TU Darmstadt in the H2Giga project PrometH2eus
In the Surface Science Laboratory at the Department of Materials and Earth Sciences, a team led by Professor Jan Philipp Hofmann is focussing on the energetics, structure and chemistry of the inner and outer surfaces of oxygen developing electrodes. These contact surfaces connect, on the one hand, the metallic electrode substrate with the active catalyst material (internal interface) for the electrolysis and, on the other hand, the electrocatalyst with the alkaline electrolytes (external interface). A special focus of the research are the electronic and chemical properties of these interfaces because this is where the critical charge transfer processes and redox reactions for the electrolysis take place. In particular, the researchers want to find out which of these processes at the interfaces limit the stability and efficiency of the electrolysis process so that they can then optimise the structure and function of the components in cooperation with their consortium partners in the next stage, also with respect to their industrial application.
Another research group headed by Professor Bastian J. M. Etzold (Technical Chemistry Group 1, Department of Chemistry) is investigating ways to improve the currently very simple geometry of nickel electrodes. Advanced grid and mesh geometry should make it possible to remove the gas bubbles that form during electrolysis more quickly and thus improve the productivity and efficiency of the electrolyser. For this purpose, the researchers are depositing nickel onto complex structured metal supports and study the advantages offered by these types of coated 3D catalysts under technical relevant conditions. The test stand being developed by this group will also act as the platform for another subproject headed by Professor Andreas Dreizler.
Professor Andreas Dreizler (Institute for Reactive Flows and Diagnostics, Department of Mechanical Engineering) and his research group are focussing on the processes related to the electrodes in an electrolyser. When electricity is applied to the electrodes, gas bubbles form, detach and then have to be removed. The researchers are carrying out experiments to investigate and optimise these multi-phase transport processes in order to test computer simulations developed by the research group headed by Dr.-Ing. Holger Marschall. In addition, the group aims to use advanced optical measuring methods for the in-operando testing of how the bubbles form and their dynamics.
The research group Computational Multiphase Flow headed by Dr.-Ing. Holger Marschall (Mathematical Modelling and Analysis Group, Department of Mathematics) is developing the digital twin to the experiments being carried out by the research group headed by Professor Andreas Dreizler. Complementary scientific questions, coordinated high-resolution experiments, and high-fidelity computer simulations are used to disclose in detail the interplay of local subprocesses and effects that influence the detachment of gas bubbles at the electrodes. The performance of an electrolyser in turn depends on the detachment behaviour of the bubbles. It will be shown how the results can later be transferred to electrolysis plants on a real scale.
Background: Green hydrogen
Green hydrogen is an energy carrier that is produced from water e.g. using electrolysis to split the water into hydrogen and oxygen. The energy required for this process comes from renewable sources such as wind or solar power, which means that green hydrogen is carbon-free and climate neutral. Green hydrogen is a secondary energy carrier. Its production and consumption are no longer bound to a particular time and place and the hydrogen can thus be saved or transported, for example.
About the hydrogen lead projects
The hydrogen lead projects represent the largest research initiative dealing with the German energy transition that has been launched to date by the German Federal Ministry for Education and Research (BMBF). In these industry-led lead projects, business and the scientific community will develop joint solutions for the German hydrogen industry with respect to the series production of large-scale electrolysers (H2Giga), the production of hydrogen at sea (H2Mare) and technologies for the transport of hydrogen (TransHyDE).
The hydrogen lead projects funded by the BMBF are the results of an ideas competition: Science, business and civil society were all invited to submit ideas for large-scale hydrogen projects. More than 240 partners are thus working together and will receive total funding of around 740 million euros. The projects were launched at the beginning of the year on the basis of non-binding letters of intent for the funding. The lead projects will be funded for a period of four years. Further information is available at


