Charge compensation mechanism in sodium cobaltate electrode materials
Electronic structure of an operando solid-state cell with sodium cobaltate cathode
2021/02/09 by J. Rohrer
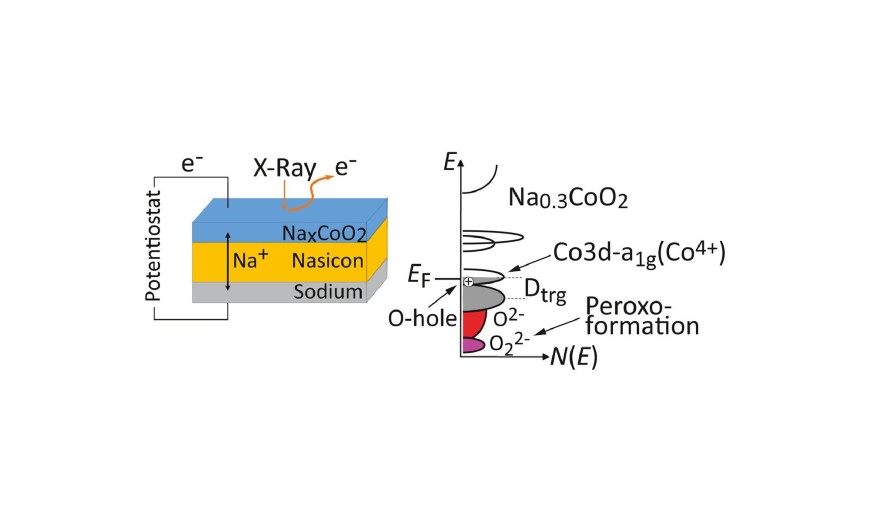
It is common belief that conventional layered oxide battery cathode materials operate on a cationic redox mechanism, and that oxygen oxidation triggered by charge compensation results in oxygen loss and limited practical capacity. In this work, thin films of sodium cobaltate demonstrate a mixed redox behavior with strong oxygen participation well within the reversible intercalation range. This unexpected behavior is attributed to an increasing degree of covalency of the transition metal-oxygen (TM-O) bonds during deintercalation in combination with a high structural flexibility of the material. These insights are based on electronic structure data obtained using an operando solid state cell, allowing analysis with unpreceded control, and are supported by density functional theory (DFT) calculations. They demonstrate that the spectrum of possible charge compensation mechanisms is broader than commonly believed, and indicate that tuning TM-O covalency is a key factor for increasing the capacity also of conventional layered materials.



